Andarine — S4, GTx-007
Andarine is a second generation Selective Androgen Receptor Modulator (SARM). SARMs are all closely related to anabolic steroids in activity.They bind the same cellular androgen receptor (AR), and impart similar anabolic effects. However, like andarine, most are non-steroidal in structure. They are also highly selective in their receptor interactions (hence the name). These drugs are often full agonists of the Aft in “anabolic” tissues such as bone and skeletal muscle, but only partial agonists in “androgenic” areas like the prostate and sex organs. As such, they should have a significantly higher separation of anabolic and androgenic effect. There is also no conversion to estrogen, and likely minimal spillover with other hormones. SARMs are intended to replicate some benefits of anabolic steroid therapy, but with fewer side effects such as prostate hypertrophy, male pattern hair loss, and virilization. Many SARMs are currently under investigation as therapeutic options for, among other things, primary and secondary hypogonadism, osteoporosis, age-related muscle wasting (sarcopenia), acquired immunodeficiency syndrome (AIDS) and cancer-related wasting (cachexia), anemia, and benign prostatic hypertrophy. Some may also turn out to be efficacious as oral male contraceptive agents. Research in this area is still early, however. Though not a developed medicine, andarine is already widely in use in the sports community as a muscle-building alternative to anabolic steroids. In animal studies, andarine has demonstrated roughly comparable anabolic effect (on muscle) as testosterone propionate. However, this came with only 30-40% of its relative androgenicity. It has also been shown to increase strength and body weight similar to dihydrotestosterone (DHT), yet is stronger at preventing and restoring bone loss. Andarine significantly decreased total fat mass of treated animals as well, which is likely attributed to its pure anabolic (non estrogenic) action. What is most critical though, at an anabolic-effective dosage, andarine provided minimal stimulation of the prostate, and only modest inhibition of the HPTA. Studies like these seem to underline andarine’s early potential as a therapeutic agent. Andarine has not been subject to full human clinical trials. Substantive data on the efficacy and potential toxicity of this drug is actually quite limited. Information on its real world effects and side effects in humans has, likewise, been largely based on observation and anecdote. So far, reports suggest that andarine does produce noticeable gains in lean muscle mass and strength.This is often accompanied by noticeable fat loss. However, it should be noted that it also appears prone to adverse reactions, particularly visual disturbances (See: Side Effects). And it is perhaps not as potent or refined as some newer SARMs. As of late, this drug seems to be losing some favor to other agents of this class.
| Brand name | Andarine, S4, GTx-007 |
Andarine Key Points
- Mild/Moderate Anabolic Effect
- Moderate HPTA Suppression
- Mild Hepatotoxicity
- Mild/Moderate Lipid Changes
- Visual Disturbances
Andarine History
Andarine was first described in 2001, in a U.S. patent filing by The University ofTennessee Research Corporation. The drug was subsequently under development by the American company GTx Inc. The earliest research on this substance was published in 2004. It used a rat model to investigate the effects of andarine on the prostate, and its potential with benign prostatic hypertrophy. GTx partnered with Ortho Biotech to develop andarine as a medicine for the treatment of cancer cachexia (wasting). The drug entered Phase I human trials, with Phase II trials planned. However, research was discontinued. This substance may have taken a back seat for more recent innovations in this space, which is now becoming quite crowded with research substances. GTx itself seems more focused on ostarine, which has been extensively studied in humans. These days, most papers on andarine look at its use in sports doping. This drug has been on the WADA banned substance list since 2008, and detectable in doping controls since 2010.
Structural Characteristics of Andarine
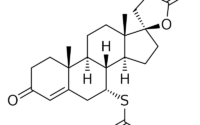
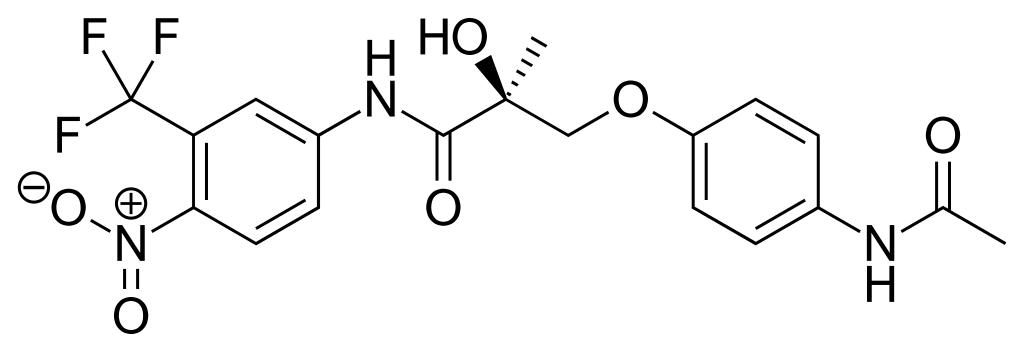
Andarine is an aryl-propionamide-derived SARM.lt is close in structure to bicalutamide, an anti-androgen. The chemical name of andarine is S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-tri-fluoromethyl-phenyl)-propionamide.
How is Andarine Supplied
Andarine is not available as a pharmaceutical product. Standard dosage information is currently unavailable. Black/gray market preparations commonly contain 25mg/ml when in liquid suspension, and 5-10 mg per capsule in oral products.
Andarine Warnings
Andarine is an unapproved new drug. A thorough understanding of its safety and propensity for side effects in humans is lacking at this time.
Andarine Side Effects
According to anecdotal reports, one of the most common side effects of andarine involves visual disturbances. This typically involves night blindness and/or a yellow tint to one’s vision. A full explanation for this side effect is lacking, though it typically resolves itself shortly after discontinuing.
This drug is also likely to negatively impact HDL (good) cholesterol levels and the HDL/LDL ratio. This may increase cardiovascular disease risk. Andarine is also mildly hepatotoxic. Liver enzymes should be monitored.
Andarine also appears to negatively influence the hypothalamic pituitary testicular axis (HPTA) similar to other anabolic/androgenic steroids, suppressing endogenous testosterone production when taken in higher doses. A traditional post-cycle therapy program is sometimes used to help restore natural hormone production more quickly after stopping use.
Andarine Administration
Andarine is given orally. This substance has not been approved for use in humans. Prescribing guidelines are unavailable.
When used for physique- or performance-enhancing purposes, Andarine is commonly used at a dosage of 25-75 mg, which is given once per day. Women usually take lower doses than men, usually opting for the low end of the range. Cycles of this drug typically last 4-8 weeks.
It is usually advised to taper-up the dosage of andarine, so that the user becomes accustomed to the effects of the drug, and is able to minimize side effects. This usually involves beginning with a 25 mg daily dose. This is increased by 10 mg every 5-7 days, until a comfortable dosage level is established.
Andarine Availability
Andarine is not available as a prescription drug product. It is sold exclusively as a “research compound” or gray market supplement. Note that the quality of gray market products like this can be difficult to assure. Care should be taken when sourcing this substance.
References
Wlliam Llewellyn (2017) – Anabolics