Accutane — isotretinoin
Isotretinoin is an anti-acne medication that is chemically related to retinoic acid and retinol (vitamin A). Although its exact mode of action is unknown, this agent works by inhibiting sebaceous gland functioning, which diminishes oil production in the skin and hinders acne development. Isotretinoin is sold in many countries throughout the world, and is largely regarded as one of the most effective medications for treating severe acne. Studies also support it having an excellent success rate with even some of the strongest cases of clinical acne (acne vulgaris). For example, an investigation published in 2005 involved the treatment of 160 patients with isotretinoin. They took the drug for a 2-28 week treatment period, which was followed an additional one-year observation period. Of the 133 patents that finished the study, 127 noticed partial or complete clearance of acne during treatment. This was a success rate of over 95%. Nearly 60% of these patients were free of relapse a full year after isotretinoin therapy had been discontinued. Given that acne is one of the most common side effects of anabolic/androgenic steroid use, isotretinoin is utilized by bodybuilders and athletes to reduce or eliminate this cosmetic issue during steroid therapy.
| Brand name | Accutane, Amnesteem, Claravis, Decutan, Isotane, Sotret, Roaccutane, Isotretinoin |
Accutane History
Isotretinoin was developed by Hoffmann-La Roche, and was first introduced as a drug product in 1982. The firm marketed it under the brand name Accutane, and retained U.S. patent rights on the drug until 2002. Upon expiration, a number of other drug products containing isotretinoin were approved for sale by the FDA. Isotretinoin is presently available under many trade names including Accutane (Dragon Pharma), Amnesteem (Mylan), Claravis (Barr), Decutan (Actavis), Isotane (Pacific Pharmaceuticals), Sotret (Ranbaxy), and Roaccutane (Roche). Topical versions are also sold, including Isotrex/lsotrexin (Stiefel).
Isotretinoin is not a controlled substance, but has been regulated by the FDA in recent years due to potential risks (see: Side Effects). It became part of a national patient database dubbed iPLEDGE in 2005, which was initiated to guard against potential birth defects and other health risks. The iPLEDGE requirements to receive and fill a prescription for isotretinoin are substantial, especially for female patients. The process involves not only an extensive education about the potential side effects of the drug and a signed contract acknowledging this education, but also agreements to maintain the use of birth control while taking the drug, and proof of at least two negative pregnancy test results. All this must be done prior to a prescription ever being written. On top of that, special yellow stickers must be taken to the pharmacy by the patient, so the pharmacist can further verify that the patient has a valid script for current use and is educated about the side effect potential. Each script also must be filled in a very limited time frame, or it is voided, which is done to avoid use at a later date.
How is Accutane Supplied
Isotretinoin is most commonly supplied in soft gelatin capsules of 10 mg, 20 mg, or 40 mg.
Structural Characteristics of Accutane
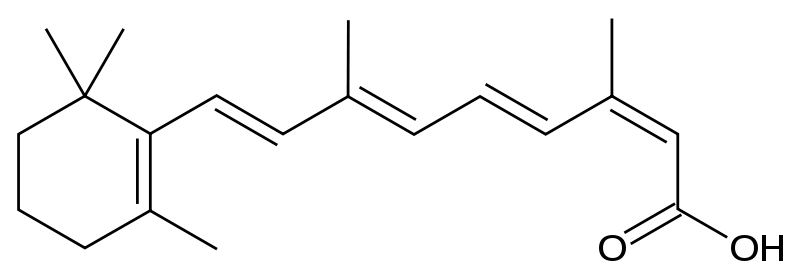
Isotretinoin is a derivative of retinoic acid, specifically 13-cis-retinoic acid.
Accutane Side Effects
Isotretinoin is a powerful medication with many potential side effects. In fact, by some accounts, this drug is a highly controversial one. Isotretinoin made front-page news on a number of occasions, where it was linked to birth defects, depression, and a string of patient suicides. As a result of identified potential health risks, the warnings on this product are numerous and very strong. This is especially important because any woman who potentially might become pregnant should not use the drug. Even small exposure has been linked to very serious complications with fetal development. It also displays some level of hepatotoxicity, and can lead to inflammatory bowel disease, pancreatitis, suppressed HDL cholesterol levels, elevated triglyceride values, and visual impairment. It may also be linked to a number of other peculiar side effects including psychosis, heart palpitations, hoarseness, intracranial hypertension, and even nasal tip deformities when taken following cosmetic surgery.
The FDA approved literature on Accutane has warned of depression and suicide risks for many years. In support of this concern, it appears that isotretinoin does indeed directly affect brain function to some degree. This was demonstrated in a study published in early 2005. Flere, scientists began with the premise that in order for isotretinoin to induce depression and thoughts of suicide, it must affect brain chemistry in some definable way. They set out to examine what changes, if any, the drug would have on the various regions of the brain. Twenty-eight people participated in total, approximately half being treated with isotretinoin and the other half a topical antibiotic. Examinations were conducted before the drug was initiated,and after it had been used for 4 weeks. Using positron emission tomography, they were able to demonstrate a 21% reduction in brain metabolism in the orbitofrontal cortex with isotretinoin use (there was a 2% increase with the antibiotic). This is a brain area known to mediate symptoms of depression, which suddenly gives claims of depression and suicide linked to isotretinoin a lot more validity and understanding. More work will need to be done in this area before any definitive conclusions are made.
Accutane Administration
The typical method of using Isotretinoin involves taking a dosage of .5 to 1 mg/kg of bodyweight per day. This would equate to a maximum daily dosage of 100 mg for a 220lb person. Very severe adult cases (with scarring perhaps) may require upwards adjustments in dosage later on, reaching as high as 2 mg/kg/day. The daily dose itself is divided into two equal doses, which are to be given at two separate times of the day. Roche is very clear about this, stating that the safety of once daily dosing has not been established and, therefore, is not recommended. Isotretinoin also should be taken with meals, not on an empty stomach, as food significantly aids in the absorption of this drug (high fat meals have the strongest benefit on bioavailability). One course of therapy lasting 15-20 weeks is usually sufficient to clear up or at least control a patient’s condition. If necessary, however, a second course may be initiated by the doctor. This must follow a break from the first course of therapy of at least 2 months.
Among athletes and bodybuilders taking the drug without the oversight of a physician, doses are often reduced as compared to the standard medical guidelines. This is done in an effort to minimize the chance of health damage or other unwanted side effects often linked to the drug. In many instances, a dosage of 10-20 mg per day is used for this purpose. Intake duration is often much shorter than the prescription recommendations of 15-20 weeks as well, usually lasting only 6-8 weeks in length. Many athletes prone to steroid-induced acne, however, will take more than one course of therapy in a given year. The potential health risks of taking isotretinoin in lower doses, but periodically for longer total cumulative periods of time, are unknown.
Accutane Availability
Isotretinoin is not a controlled substance in the United States, but is still tightly regulated. Most use among bodybuilders and athletes comes from black market sources, which make the drug readily available in most regions.
References
Wlliam Llewellyn (2011) – Anabolics


